Medical Device Regulation (MDR)
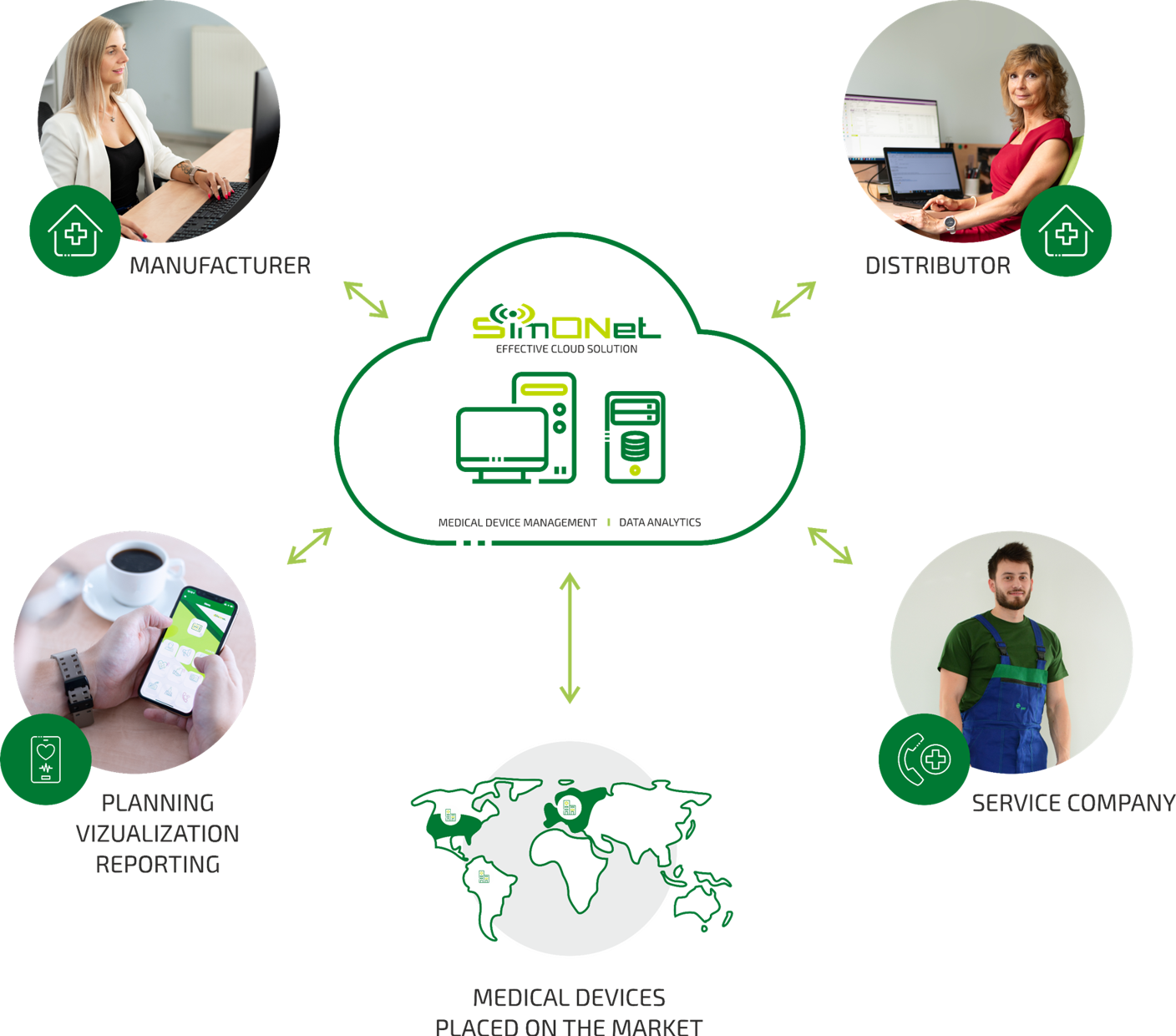
MDR (Medical Device Regulation) – is a demanding new legislative requirement of the European Union. This regulation applies to manufacturers, distributors, service organizations, and users of medical devices on the EU market. The aim of this regulatory framework is to increase the safety and quality of medical devices within the European Union.
MDR has brought a number of challenges for medical device manufacturers, including the mandatory implementation of a Post-Market Surveillance (PMS) system. This involves continuous monitoring and evaluation of the technical parameters, safety, and performance of medical devices after they have been placed on the market.
SimONet MDR Solution
SimONet MDR Solution is a cloud-based platform for efficient Post-Market Surveillance (PMS) that simplifies processes and ensures compliance with evolving MDR requirements. It automates data collection, risk analysis, and documentation updates. The platform centralizes surveillance tasks, enhances traceability, supports complaint review, and strengthens cybersecurity.
This allows manufacturers to effectively meet MDR obligations, including PSUR and PMCF, and ensure the long-term safety of their products.
- Acceleration of workflows in the ever-evolving MDR regulatory environment
- Online tiered access to up-to-date and accurate information
- All surveillance activities and tasks in one place
- Review of customer complaints and adverse events, including CAPA (Corrective and Preventive Actions)
- Traceability and efficient maintenance of supervised medical devices

Compliance with cybersecurity requirements

Continuous data collection and analysis

Easy compliance with inspections and audits requirements

Compliance with PSUR and PMCF requirements
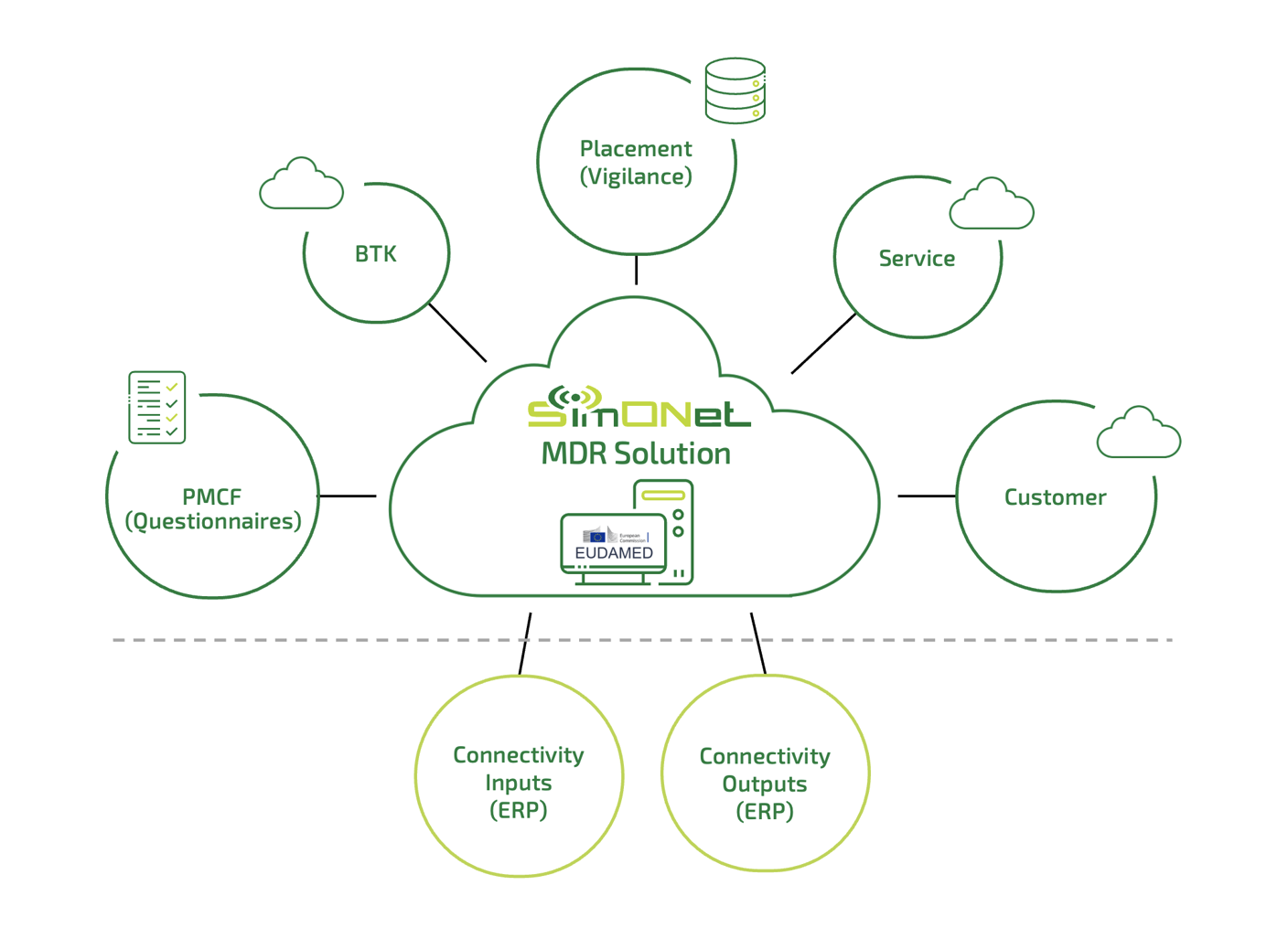
SimONet MDR modules
Placement of medical devices (Vigilance) – market surveillance showing the location of each medical device on the market, with visual reporting of device placement.
PMCF (Questionnaires) – collection and evaluation of data based on customer requirements (same principle as the BTK Module, but with different data content). A universal module fundamentally based on the BTK module (different values in tables, different report content). This involves mandatory anonymized data collection from medical devices, e.g., questionnaire surveys (Pain Map, Pain Intensity, Oswestry, DCB, SF-MPQ…).
Customer – managerial views across all modules, graphs, customer customization – colors, logos, visualization, other customer data not included in the mentioned modules.
BTK – medical device records, monitoring, maintenance scheduling, and output reporting.
Service – evidence of medical devices, monitoring, scheduling of services, prediction of service interventions.
Connectivity Inputs (ERP), Outputs (ERP) – customer connectors for integration with ERP systems.
Want to know more? Contact us!



